
IROSE
News on Desalination
Department of Energy Desalination contest
Amazon rain forest health All files Desal.210_video, Video-ytub, http://jontalle.web.engr.illinois.edu/MISC/Desalination.21/00_VideosDesal-Jul26.21/Overview-System,
Allen Presentations
presentations (pdf)
Discussion the solution:
Water is a local problem and it needs local solutions, said Priyanka Jamwal, a fellow at the Ashoka Trust for Research in Ecology and the Environment in Bangalore (See World water stress). But to solve this world wide crisis we need to go where the water is, namely the ocean. But you will note: the ocean is saltwater. We need fresh water. Desalination plants around the world are base on reverse osmosis (RO), an expensive resource that is not ideal to the demands of fresh water. The oceans are become more acidic due to the build up of CO2, which is highly soluble in water. In fact much of the CO2 generated by our burning fossil fuels ends up in the ocean where it acidifies the ocean, killing the coral, and thus the life in the ocean, one of our main sources of food. Of course the main problem is the population on the earth has created these many intertwined problems, and if we don't reduce the population, it will be reduced the hard way. If we could find a better method that RO, perhaps we could solve the water shortage problem, once and for all. In the mean time we need to reduce the population and the build up of CO2 and other green house gases.
Where does rain come from? The sun evaporates the surface water from the ocean, forming clouds, which eventually dump the moisture as rain. Can this process be captured under our control so that we can make rain as needed rather than depending on natural processes, which are random unreliable sources of water? In this presentation I shall present a scenario for how to convert sea water into fresh water, and at the same time extract the CO2 and convert it into graphite, directly from the energy from the sun, using the same process used by nature.
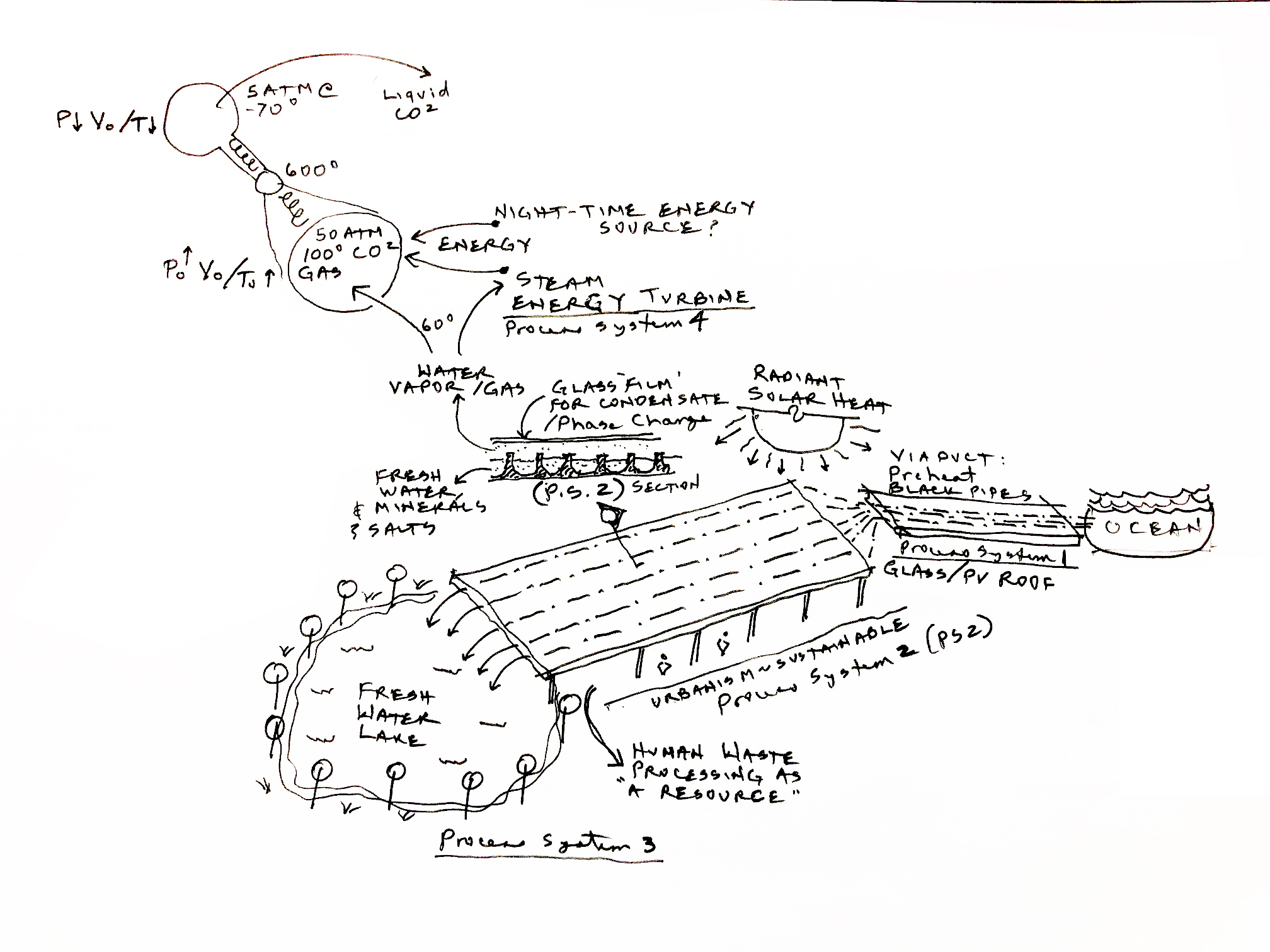
The above figure is a proposal on how to solve the many world stress problems. It starts with a wide (perhaps hundreds of meters) and very (thousands of meters) long aquifers, which we call aquipures, into which sea water is pumped in large quantities. The aqupures then transport the seawater to remote desert locations. A related idea, but based on RO has been implemented in Aba Daubi. But there much more to this proposal, which is not based on RO.
On the way to the final destination of the aquipure the sunshine evaporates the water, converting into water vapor (think high humidity). To trap the water vapor the aquipures are covered by a thin sheet of plastic, transparent to the sun's light. The water in the aquipures is continuously vaporized into and aerosol (small sub-millimeter sized droplets, to greatly accelerate the evaporation, by increasing the water's surface.
Each of these processes only take small amounts of electricity (compared to that required for RO desalination). Once the humidity is raised to close to 100%, the moist air is sucked down channels by a low vacuum, where it comes in contact with a chilled surface. The cooling of the surface could be done by the ocean water as it comes in from the sea, which is typically much colder than the air. The slightly warmed sea water would then be used to flush the concentrated brine, resulting from the removal of H2O and CO2 from the sea water.
There are several scenarios for separating the CO2 from the sea water or from fresh water. CO2 is twice the mass of H20, thus centimeter size very high speed rotating centrifuges would naturally separate the two liquids, or gases. Such a process is used today to separate uranium 235 from 237. In that case the mass difference is slightly less than 1%, so the 2:1 ratio of CO2 over H2O can be much more efficient. The separation is enhanced by rotating the centrifuge at a high speed, increasing the radial forces on the molecules.
The above methods needs to be tested on a small scale, and once the many logistic details are worked, deployed on a mass scale. Over the last hundred years it is said that about 1/3 tera-ton of CO2 has been released into the environment by various human activities. It seems likely that it will take all the energy we generated from the fossil fuels, and more, to now remove them. Perhaps the energy from the sun is insufficient to do the job. In this case we will need additional energy from modern nuclear reactors. This new technology is much safer and smaller in scale, making it a practical option to solar. One source (April 13, 2013) claimed these reactors would be cheaper and cleaner than natural gas energy
Desalination senerios:
Process senerios:
- There are a number of process scenarios (PS-1, PS-2, ...) in play here:
- PS-1: Ocean water is pumped into the aquifer exposed to the sun.
- PS-2: Above the brine is a transparent cover as shown. The heat of the sun evaporates the water, raising the humidity. The humid air is then driven by convection onto a ocean chilled plate where the vapor condenses into liquid water. This process concentrates the \(CO_2\) in the brine.
- PS-3: The desalinated water is then fed to the lake at the far end of the aquifer. This is used to grow plants and support wildlife.
- PS-4: Assuming that the \(CO_2\) has been isolated it is reduced to \(O_2\) and carbon. There seem to be a number of methods for doing the seperation. This requires power during the night, requiring a nuclear reactor. Today there are low-power (50-100 MW) and much safer versions available. If buried in the ground they can be made free from a terror attack (See Fussion methods).
Resources
- Decarbonization methods: \(CO_2\) removal
- Direct capture of CO2 from sea water: Seawater capture is akin to direct air capture, except CO2 is extracted from seawater instead of air. By reducing CO2 concentration in the ocean, the water then draws in more carbon from the air to regain balance. Seawater is a more concentrated solution of CO2 than the ambient air, which means less work is required to separateit out than in direct air capture.
- Gas to solid: Some minerals naturally react with CO2, turning carbon from a gas into a solid. The process is commonly referred to as “weathering,” and it typically happens very slowly—on a geological timescale. But scientists are figuring out how to speed up the process, especially by enhancing the exposure of these minerals to CO2 in the air or ocean. That could mean pumping alkaline spring water from underground to the surface where minerals can react with the air; moving air through large deposits of mine tailings—rocks left over from mining operations—that contain the right mineral composition; crushing or developing enzymes that chew up mineral deposits to increase their surface area.
- NOAA: NOAA data, CCIS workshop
- Fussion methods: SMR: no CO2, small modular reactors, Safe small nuclear, Milli-Fission: 50 MW which projects same cost as natural gas
- Reverse Osmosis (RO) methods: Large scale RO, San Diego plant, Abu Dhabi desert rebuild plan
- Water properties: How to calculate due point, Psychrometric chart and water vapor
- Politics and comment: clean cars a must, Political action committee, Our ocean health
- Opinion: Opinion Al Gore, electrolysis
- Ocean temperature
- The amount of carbon dioxide released into the air reached an all-time high in 2018, weighing in at 37.1 billion tonnes of the planet-warming gas. Fortunately, through the carbon-capturing abilities of the world’s oceans, about a quarter of it was drawn underwater, where its greenhouse gas effects are put on hold. Increasingly, we’re relying on the oceans as a crutch, but as new research from Columbia University’s Earth Institute reveals, they soon may not be able to store carbon like they used to.
Powered by PmWiki